Stainless steel is a type of metal material resistant to corrosion, but during the manufacturing process, it undergoes procedures such as transportation, marking, cutting, forming, and MIG welding, which may result in scratches, marks, and hammer imprints on the surface. These defects decrease the corrosion resistance of the components, directly impacting their lifespan. Passivation, however, is a method to transform the surface of stainless steel into a state less susceptible to oxidation, thereby slowing down its corrosion rate.
In this article, we will delve into when stainless steel requires passivation and what passivation entails.
What is Stainless Steel Passivation?

Passivation of stainless steel is a surface finishing method aimed at transforming the surface into a state less prone to oxidation, thus slowing down its corrosion rate. This is achieved by forming an extremely thin layer of chromium oxide (Cr2O3) on the exposed surface of the stainless steel. When the stainless steel surface comes into contact with oxygen in the air, chromium reacts with oxygen to form a dense chromium oxide film. This oxide film can block oxygen, water, and other corrosive media in the external environment, protecting the stainless steel surface from corrosion.
Common Corrosion Types of Stainless Steel
Stainless steel is not easy to rust steel(To learn about the difference between steel and stainless steel, please refer to this article:https://www.boyiprototyping.com/materials-guide/alloy-steel-vs-stainless-steel/), the main alloying element in stainless steel is Cr (chromium), only when the Cr content reaches a certain value, the steel has corrosion resistance, stainless steel generally Cr content of at least 10.5%. The corrosion resistance mechanism of stainless steel is the passivation film theory, that is, the surface forms an extremely thin and solid and fine stable Cr-rich passivation film, preventing oxygen atoms from continuing to penetrate and continue to oxidize, so as to achieve the ability to prevent rust.
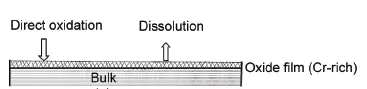
People think that “stainless steel is not rust, rust is not stainless steel.” In fact, this is a one-sided wrong view of the lack of understanding of stainless steel, stainless steel will rust under certain conditions. If we can intuitively understand the various corrosion types of stainless steel, we can have corresponding countermeasures to reduce losses in the face of stainless steel corrosion.
Corrosion damage of stainless steel is mostly local corrosion damage, the most common are intergranular corrosion (9%), pitting corrosion (23%) and stress corrosion (49%).
1.Intergranular Corrosion

Intergranular corrosion is a form of localized corrosion that mainly occurs at the interfaces between metal grains and extends inward along these boundaries. The occurrence of intergranular corrosion is closely related to the temperature control of stainless steel during heat treatment. In the temperature range of 450 ℃ to 850 ℃, C and Cr are prone to form carbon chromium compounds (Cr23C6). When the Cr consumed at the grain boundary cannot be replenished from the grain in a timely manner, a Cr deficiency phenomenon will occur in the grain boundary area, leading to intergranular corrosion.
To prevent intergranular corrosion of stainless steel, the general solution is:
- The solution annealed metal is uniformly heated to 1050 ℃~1060 ℃, and then rapidly cooled;
- Add stabilizing elements such as Ti and Nb;
- Choose low-carbon stainless steel.
2.Pitting

Pitting corrosion is a highly destructive form of localized corrosion, typically characterized by small yet deep pits on the metal surface. Once formed, these pits accelerate the corrosion process dramatically and can even lead to perforation, posing a serious threat to the safety and lifespan of equipment.
For instance, in daily life, stainless steel sinks (mostly made of 201 or 304 stainless steel) often experience pitting corrosion. If acidic or salty substances are left untreated in the sink, it can result in pitting corrosion of the stainless steel sink.
Preventive measures include:
- Preventing Cl- attachment.
- Conducting proper surface treatments to form stable passive films.
- Choosing materials with strong resistance to Cl- corrosion, such as Mo-added 316L stainless steel.
3.Stress Corrosion

Stress corrosion is a destructive phenomenon that occurs when metal is subjected to tensile stress and a corrosive environment simultaneously. For stainless steel, stress corrosion is one of the most frequent and severe forms of corrosion leading to failure. The characteristic feature of this corrosion type is its rapid propagation rate once slight cracks appear, potentially resulting in catastrophic consequences. Stress corrosion cracking of stainless steel materials is common in industrial environments such as chemical plants, nuclear power stations, and boilers.
To prevent and address stress corrosion in stainless steel, the following measures can be taken:
- Reduce stress concentration in high-stress components through proper structural design and manufacturing processes.
- Prevent the introduction of chloride ions, as they are one of the main factors contributing to stress corrosion in stainless steel.
- Avoid processes that induce stress, such as cold working, as they may increase the risk of stress corrosion.
- Utilize heat treatment to eliminate or alleviate residual stresses in the material.
- Select stainless steel materials with good resistance to stress corrosion, such as ferritic stainless steel or high-Ni alloys.
Why Does Stainless Steel Require Passivation?
The main reason stainless steel requires passivation treatment is to enhance its corrosion resistance, chemical stability, and aesthetic appeal. While stainless steel exhibits relatively good rust resistance, in coastal areas or environments exposed to certain acidic or alkaline agents, chloride ions can easily penetrate the passivation film of stainless steel. Over time, stainless steel can gradually corrode and rust. Therefore, passivation treatment is necessary for stainless steel. Passivated stainless steel can extend its rust resistance by 3 to 8 years on top of its inherent corrosion resistance, significantly reducing the likelihood of rusting.
Here are the benefits of passivation treatment for stainless steel:
- Enhanced Corrosion Resistance: Passivation treatment forms a dense oxide film on the surface of stainless steel, effectively isolating it from direct contact with the external environment. This film acts as a barrier against external corrosive factors, such as oxygen, water, or other corrosive media in natural environments. The dense oxide film prevents easy penetration, significantly boosting the corrosion resistance of stainless steel and thereby extending its lifespan.
- Improved Chemical Stability: The passivation layer not only provides physical protection but also forms a chemically stable bond with the substrate. This bonding creates a smoother and more uniform surface, reducing the formation of minor defects and cracks, thus further enhancing its chemical stability.
- Enhanced Aesthetic Appeal: Passivated stainless steel surfaces exhibit a more uniform and smoother appearance, enhancing their aesthetic appeal and decorative effect. This improvement not only increases the attractiveness of stainless steel products but also enhances their value and competitiveness in the market.
Put your parts into production today
All uploads are secure and confidential.
When is Passivation of Stainless Steel Required?
Stainless steel requires passivation treatment in the following situations:
1.After Welding
Welding disrupts the passivation film on the surface of stainless steel, leading to a loss of some corrosion resistance. Hence, stainless steel after welding may need to undergo passivation treatment to restore its corrosion resistance.
You can refer to this article to understand what good welding and bad welding are:
Bad Welding vs Good Welding: Details Explained
2.Exposure to Harsh Environments
Stainless steel exposed to harsh environments such as coastal areas or highly polluted regions may face increased risk of corrosion and rust due to corrosive substances like chloride ions, which can easily penetrate the natural passivation film on stainless steel. Therefore, stainless steel used in such environments requires passivation treatment to enhance its corrosion resistance.
3.Contact with Corrosive Substances
Stainless steel surfaces that frequently come into contact with acidic or alkaline agents or other corrosive substances are prone to erosion. Passivation treatment forms a more stable and dense passivation film, preventing direct contact between these corrosive substances and the stainless steel substrate.
4.Aesthetic Requirements
Passivation treatment enhances the brightness and aesthetics of the stainless steel surface, making it appear more attractive and uniform. This makes it suitable for applications where aesthetic standards are high.

Principle of Passivation for Stainless Steel is What?
The principle of passivation treatment for stainless steel can be explained using the thin film theory. This process involves a chemical reaction between the surface of the stainless steel and the passivating agent. When stainless steel is immersed in a solution containing a passivating agent, the metal elements on its surface react with the oxidizing agents present in the passivating agent. This reaction results in the formation of an extremely thin, dense, and uniform passivation film on the surface of the stainless steel.
This passivation film is primarily composed of oxides or hydroxides and exhibits high corrosion resistance and mechanical strength. It adheres to the stainless steel surface, forming a barrier that completely isolates the metal from external corrosive media such as oxygen, moisture, and harmful chemicals. By preventing direct contact between these corrosive media and the stainless steel substrate, the passivation film effectively inhibits further dissolution and oxidation of the metal, thereby preventing corrosion and rust.
It plays a role in completely separating the metal from the corrosive medium, preventing direct contact between the metal and the corrosive medium, thereby preventing the metal from dissolving and forming a passive state, achieving the purpose of corrosion prevention and rust prevention. Acid pickling and passivation can quickly pickling the surface welding spots and seams of stainless steel materials, improving the surface rust resistance and salt spray time of the product.
Stainless Steel Passivation Process: Step-By-Step Explanation
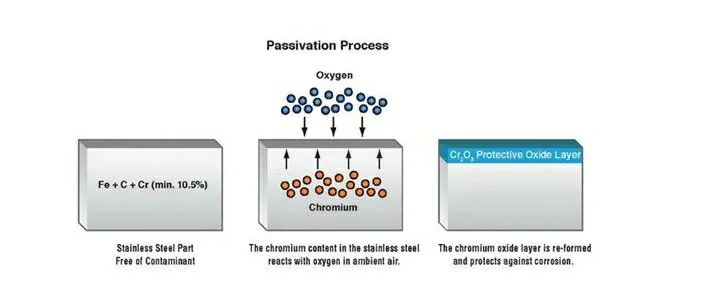
The stainless steel passivation process mainly includes the following steps:
1.Cleaning:
The primary objective is to clean the surface of stainless steel, removing oil, dust, and oxides. There are various methods available for degreasing stainless steel surfaces. Alkaline detergents and high-temperature baths (up to 65°C) are highly effective for dissolving and removing impurities. The specific method depends on the level of contamination on the stainless steel surface.
2.Pickling:
After cleaning, the stainless steel surface needs to undergo pickling to further remove oxides and other impurities. Common pickling agents include nitric acid, sulfuric acid, and citric acid(the next section will provide a detailed introduction to the functions of these three pickling agents). It’s essential to control the time, acid concentration, and temperature during the pickling process to ensure effective removal of contaminants without causing excessive corrosion to the stainless steel substrate.
3.Passivation Treatment:
Passivation treatment typically involves immersion of stainless steel materials in a solution containing passivating agents for a certain period. Passivating agents react chemically with the stainless steel surface, forming a dense passive film. This passive film effectively enhances the corrosion resistance of stainless steel.
4.Cleaning and Drying:
After passivation treatment, thorough cleaning is necessary to remove any residues of passivating agents. Once cleaning is complete, the stainless steel surface should be dried to prevent oxidation or corrosion. Drying ensures the stainless steel surface remains dry and smooth, minimizing the risk of subsequent oxidation or corrosion.
5.Quality Inspection
Appearance inspection: The surface of stainless steel pickling and passivation should be uniformly silver white, smooth and beautiful, without obvious corrosion marks. The weld and heat affected zone should not have oxidation color, and there should be no uneven color spots.
Residual liquid inspection: Use phenolphthalein test paper to check the cleanliness of residual liquid on the surface of stainless steel. If the pH value is neutral, it is considered qualified.
Blue dot test: The basic principle of the blue dot test method is that if the surface passivation film is incomplete or contaminated with iron ions, free iron ions will exist, and potassium ferrocyanide solution will react with iron ions to form a blue precipitate.
Put your parts into production today
All uploads are secure and confidential.
Stainless Steel Passivation – Pickling Agent Type
Industry standard passivation methods for stainless steel surfaces: nitric acid, nitric acid with sodium dichromate, citric acid. Each type relies on specific chemicals for passivation treatment to meet the requirements of different environments and applications.
Nitric Acid Passivation: By immersing stainless steel components in a solution containing a specific concentration of nitric acid, a dense passivation film can form on the stainless steel surface. This passivation film can enhance the corrosion resistance of stainless steel, protecting its substrate from oxidation corrosion.
Nitric Acid with Sodium Dichromate Passivation: The addition of sodium dichromate can further improve the quality and corrosion resistance of the passivation film. This passivation method is typically suitable for stainless steel components with higher corrosion resistance requirements. It’s important to note that sodium dichromate is a hazardous substance, and its concentration and handling conditions need to be strictly controlled during use.
Citric Acid Passivation: Citric acid has the advantages of being environmentally friendly and non-toxic. Citric acid can react with oxides on the stainless steel surface, remove oxide layers, and form a thin and dense passivation film on the surface. Citric acid passivation typically involves both pickling and passivation treatment steps. By controlling the treatment time and temperature, excellent passivation results can be achieved.
Special Precautions for Stainless Steel Passivation
Before passivation, the surface of stainless steel must be thoroughly cleaned to remove all grease, dirt, and other foreign matter. The presence of grease and other contaminants can affect the quality of acid passivation. During cleaning, alkaline detergents or other suitable cleaning agents can be used.
The chloride ion content in the pickling solution, passivation paste, and cleaning water must be strictly controlled. Excessive chloride ion content can damage the passivation film of stainless steel, thereby reducing its corrosion resistance. For example, in the ship standard “Stainless Steel Pickling Passivation Paste” CB/T3595-94, the chloride ion content of stainless steel pickling passivation paste should be controlled within a specific range, generally required to be between 25ppm and 100ppm. For cleaning water, the chloride ion content should also be controlled at levels below 25PPM.
The waste liquid generated during the pickling and passivation process must be properly treated to comply with national environmental emission requirements. The waste liquid needs to be neutralized before discharge. For example, for fluoride-containing waste liquid, hydrated lime or calcium chloride can be added for treatment; for chromium-containing waste liquid, ferrous sulfate can be added for reduction treatment. These treatment measures can effectively reduce the content of harmful substances in the waste liquid and protect the environment.
During the passivation process, operators must wear appropriate personal protective equipment, such as protective goggles, gloves, and protective clothing, to prevent contact between the solution and the skin. If passivation solution splashes into the eyes or onto the skin accidentally, it should be immediately rinsed with clean water, and medical assistance should be sought.
Difference Between Passivation vs Rust Prevention Oil
The main difference between passivation and rust prevention oil is that the generated products are different; Anti rust oil is a physical reaction that effectively prevents rust by sealing the pores on the metal surface with an oil film to isolate contact with oxygen. The oil film is more prone to failure due to being removed and damaged as production progresses; Passivation, on the other hand, utilizes the oxidizing substances in the passivation solution to undergo an oxidation-reduction reaction with the metal, promoting the formation of a layer of metal oxide compounds on the metal surface and achieving effective protection of the metal. This process belongs to a chemical reaction. The resulting passivation film is dense, intact, and not easily damaged.
Summary
In conclusion, passivation is highly necessary for stainless steel products intended for use in special environments. Through passivation treatment, not only can the corrosion resistance and durability of products be significantly improved, ensuring their long-term stable operation in harsh environments, but also the appearance quality of products can be maintained, enhancing their overall performance and value.
As a professional manufacturing service provider, Bo Yi has extensive experience and advanced technology to offer comprehensive stainless steel passivation solutions to our clients. With our professional team and advanced equipment, we can ensure the quality and efficiency of passivation treatment. Whether it’s for different materials of stainless steel or various application scenarios, we can provide customized passivation services to ensure the best performance and appearance of products.
Put your parts into production today
All uploads are secure and confidential.
If you need more information about stainless steel passivation or want to learn about our manufacturing services, please feel free to contact us. We will provide you with professional answers and high-quality services to help you solve all issues related to stainless steel passivation.
FAQ
The most commonly used chemical for passivating stainless steel is nitric acid. Nitric acid passivation removes any free iron from the surface of the stainless steel, creating a thin, protective oxide layer that enhances its corrosion resistance. This process is often referred to as chemical passivation or pickling.
QQ-P-35 is a military specification outlining requirements for the passivation of stainless steel parts, while ASTM A967 is a standard developed by ASTM International covering passivation of stainless steel with a broader scope.
The duration of passivation for stainless steel can vary based on factors like environmental conditions and maintenance practices. Generally, a well-done passivation treatment can provide lasting corrosion resistance, often several years, but regular maintenance may be needed to ensure prolonged effectiveness.



